It contains an a amino group an a carboxylic acid group and a side chain consisting of a 3 carbon aliphatic straight chain ending in a guanidino group. Learn vocabulary terms and more with flashcards games and other study tools.
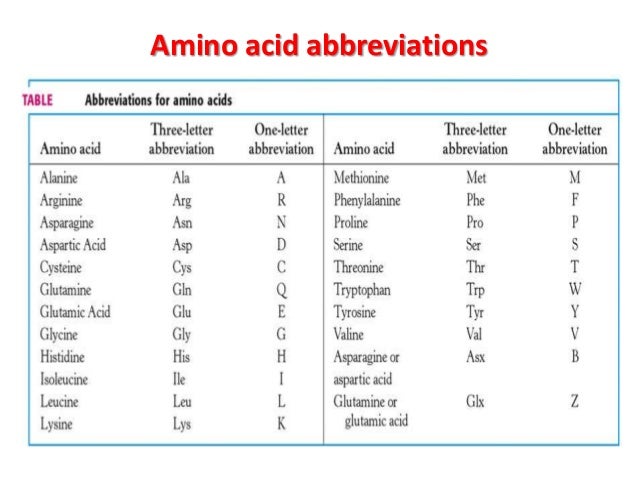
Chemistry Of Amino Acids Proteins

Redacted Acidic Amino Acids Have Negative Charge At Physiological Ph
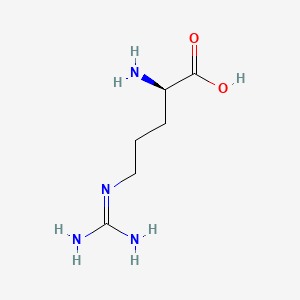
D Arginine C6h14n4o2 Pubchem
How do you calculate the net charge on the amino acid alanine at ph of 8 if the following pk values are given.

Arginine charge at ph 8. Negative charged acidic side chains. Arginine has a net charge of 083 at ph 132 and am supposed to support this using the h h equation. The charge on the amino acid side chain depends on the pk of the aa table 1 and on the ph of the solution.
There are three amino acids that have basic side chains at neutral ph. Their side chains contain nitrogen and resemble ammonia which is a base. As the ph rises to between 18 to 6 the amino acid has a net positive charge as carboxylic acid group becomes deprotonated and the terminal.
Amino acids can exist as zwitterions containing a protonated amine group and deprotonated carboxyl group. Similarly pk a is the negative log of the acid dissociation constant for an amino acid side chain. Amino group has a pka of about 9 10 so the amino group will also be deprotonated 0 charge lysines side chain amino group at the end of its side chain has a pka of about 9 10 so at ph 14 it will be deprotonated 0 charge therefore the net charge on lysine at ph14 is 1 0 0 1 by similar reasoning at physiological ph the net.
The pi or isoelectric point corresponding to the zwitterion form lets you calculate the ph at which an amino acid will have a net zero charge. Log in arginine. However i am not getting the right answer.
Lets examine this with the amino acid histidine. These are arginine arg lysine lys and histidine his. Ph is the negative log of the hydrogen ion concentration in a solution.
Amino acids side chain charge at ph 74. Relationship of charge to ph. Arginine also known as l arginine symbol arg or r is an a amino acid that is used in the biosynthesis of proteins.
Aspartic acid and glutamic acid at a ph superior to their pk table 2 the carboxylic side chains lose an h ion proton and are negative charged. Side chain charge at ph 74. Their pkas are high enough that they tend to bind protons gaining a positive charge in the process.
In solution if ph pk a then the protonated form of an amino acid side chain predominates according to the henderson hasselbalch equationif ph pk a then the deprotonated form of the amino. Learn vocabulary terms and more with flashcards games and other study tools.
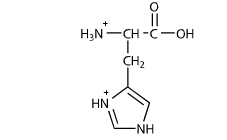
Histidine Charge At Ph 7 Student Doctor Network

Midterm Review 1 2017 Questions And Answers Bch 100 Studocu

Improving Mass Spectrometry Analysis Of Protein Structures With

EmoticonEmoticon