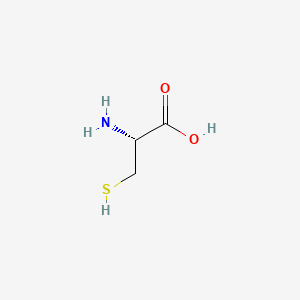
Cysteine containing peptides when peptides are required for antibody production a cysteine can be added to the n or c terminus. The cysteine sulfhydryl group plays a crucial role in the synthesis of large peptides by.
Fmoc Cys Trt Oh 103213 32 7 Discovery Fine Chemicals
Custom Cyclic Peptide Synthesis With Disulfide Bond
Cysteine C3h7no2s Pubchem
Peptides with this modification are mainly used in peptide mass fingerprinting for identification and characterization of proteins.

Cysteine peptide synthesis. The key step of ncl consists of the reaction of a peptide thioester with a peptide contain ing an n terminal cys. A variety of fluorescent dyes can be coupled to peptides. The role of cys in native chemical ligation.
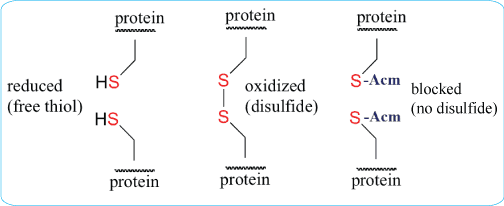
The synthesis of a peptide acid containing a c terminal cysteine residue require special consideration as extensive epimerization 4 and b piperidinylalanine formation 5 can occur during chain extension. Native chemical ligation ncl 1415. Dtnp and dtp are solids and have good solubility in tfa and can thus be added to any existing cleavage cocktail.
Biotinylated peptides can be obtained by reaction of iodoacetyl biotin with the cysteine side chain thiol. The dpra measures the reaction of a chemical with synthetic peptides containing cysteine ac rfaacaa cooh or lysine ac rfaakaa cooh to assess its sensitisation potency. Cysteine peptide for dpra direct peptide reactivity assay for skin sensitisation testing.
The role of scavengers in cleavage cocktail since the development of fmoc based solid phase peptide synthesis a wide variety of cleavage cocktails have emerged. I have recently synthesised some short around 20mer peptides containing between 2 and 4 cysteines but i cant seem to dissolve them. We have presented in this paper an extremely facile and gentle method to remove mob and acm protecting groups from cysteine and selenocysteine after completion of solid phase peptide synthesis.
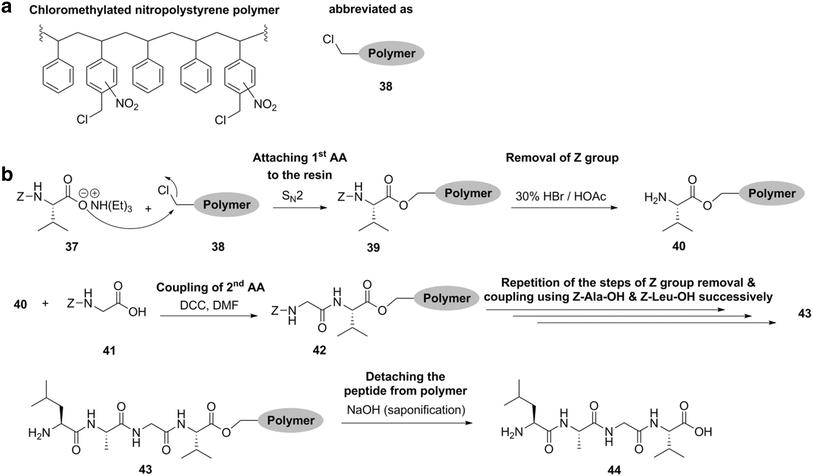
Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Carbamidomethylation cam is a deliberate post translational modification introduced to cysteine residues by reacting with iodoacetamide. In organic chemistry peptide synthesis is the production of peptides compounds where multiple amino acids are linked via amide bonds also known as peptide bonds.
These side reactions are most problematic where the cysteine residue is anchored to a wang type resin. When incorporating a cysteine residue at the peptide terminus the addition of a flexible amino acid linker such as glycine serine dipeptide between the peptide and terminal cysteine residue provides a convenient spacer that can reduce interference between the functional region of the polypeptide and the dna polymer. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains.
How to dissolve cysteine containing peptides. Chemical peptide synthesis most commonly starts at the carboxyl end of.

News In Proteomics Research Iniparib Nonselectively Modifies
Thirteen Decades Of Peptide Synthesis Key Developments In Solid

Peptide Design Thermo Fisher Scientific Us

EmoticonEmoticon