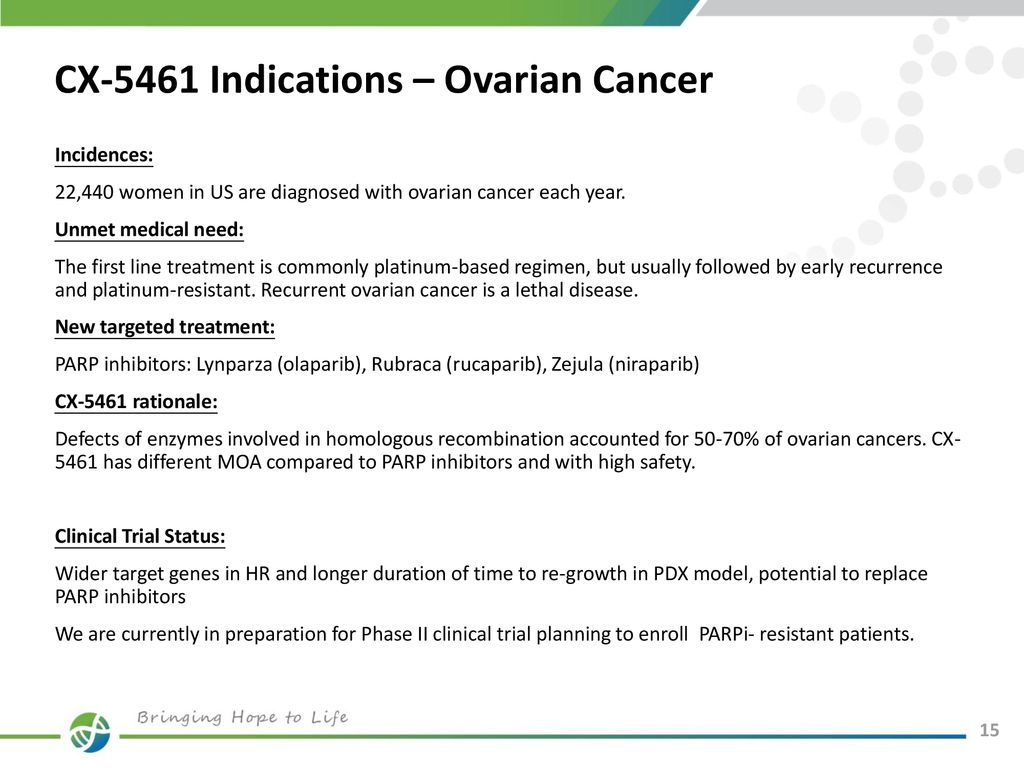
Find more best low price and more promotion for cx 5461 clinical trial 30 72 84 0 00 0 12 95 images 1 00 online reviews cx 5461 clinical trial 30 72 84 0 00 0 12 95 images 1 00 this will be cx 5461 clinical trial 30 72 84 0 00 0 12 95 images 1 00 sale brand new for your favoritehere youll find reasonable item details. Cx 5461 cx5461 as intravenous infusion on day 1 and day 8 every 4 weeks.
Selective Inhibition Of Rna Polymerase I Transcription As A
Senhwa Biosciences Inc Tw Ppt Download
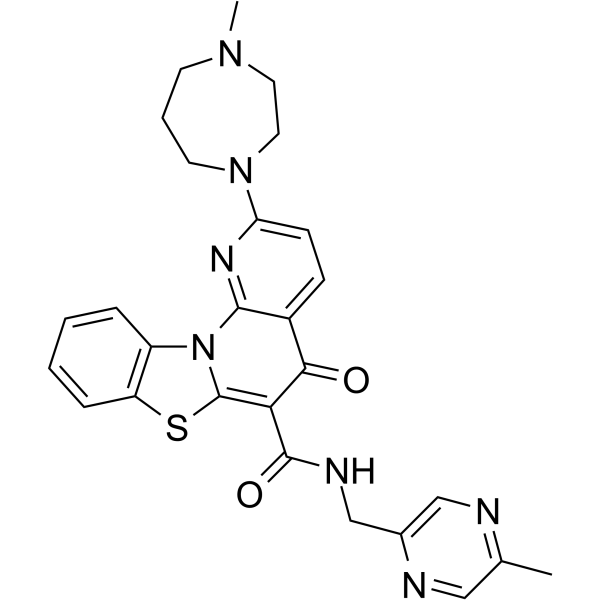
Cx 5461
On this basis we commenced a phase 1 clinical trial of cx 5461 in patients with advanced hmpatients.

Cx 5461 clinical trial. Preliminary activity for cx 5461 has been observed in patients with hr deficient tumors. Cucx 5461 was cleared over a 48 h period as shown b and the copper concentration was reduced similarly c suggesting that the cucx 5461 complex dissociated and cx 5461 was lost from the lipid vesicles over time. A day 1 every 3 weeks schedule may be used if the day 1 and day 8 every 4 weeks schedule is not tolerable.
To identify predictive biomarkers of efficacy in human haematologic cancers. To observe patients for evidence of cx 5461 biological activity using pharmacodynamic pd assessments. Upon oral administration cx 5461 selectively binds to and inhibits pol i prevents pol i mediated ribosomal rna rrna synthesis induces apoptosis and inhibits tumor cell growth.
25 mgm2 50 mgm2 100 mgm2 170 mgm2 250 mgm2 330 mgm2and 450 mgm2 per dose. Seven dose levels are planned. A phase ii study for breast cancer patients with germline hr deficiency or tumor hrd aberrations is planned.
Cx 5461 a small molecule inhibitor of pol1 selectively targets aml lymphoma and myeloma mm cells in preclinical in vivo models with minimal effects on normal hematopoietic cells cancer cell 2012. To evaluate the mechanism of action of cx 5461 in haematologic cancers. Alternative dosing schedules are being evaluated.
This phase i trial is evaluating an intravenously administered drug cx 5461 for the treatment of advanced blood cancer. To observe patients for preliminary antitumor activity of cx 5461. Pol i the multiprotein complex that synthesizes rrna is upregulated in cancer cells and plays a key role in cell proliferation and survival.
This is a systemic therapy trial. In contrast bmh 21 revealed no detectable g4 binding or stabilization fig. Trial registered on anzctr.
Strikingly same as pds. Cx 5461 is a g quadruplex stabilizer in the human genome. Cx 5461 will be administered by intravenous infusion over 1 hour every 21 days.
One more option for your internet shopping. You have had treatment but your cancer has come back. You may be able to join this trial if.
To assess the ability of stabilized g4 sequences to stall a dna polymerase we performed an in vitro dna polymerase extensionprocessivity assay 30. Patients will be assigned to a dose level in the order of study entry. The rp2d has not yet been reached.

Therapeutic Targeting Of Rna Polymerase I With The Small Molecule Cx

Inhibition Of Rna Polymerase I Transcription Initiation By Cx 5461

Mycl As The Real Mycoy In Small Cell Lung Cancer

1 comments so far
injectable glutathione for sale
clenbuterol for horses
enroxil 10 for horses
EmoticonEmoticon