At ph7 two are negative charged. What is the overall charge positive negative or neutral of arginine with a ph of 1 7 and 10.
Solved Calculate The Average Charge On Arginine When Ph 9

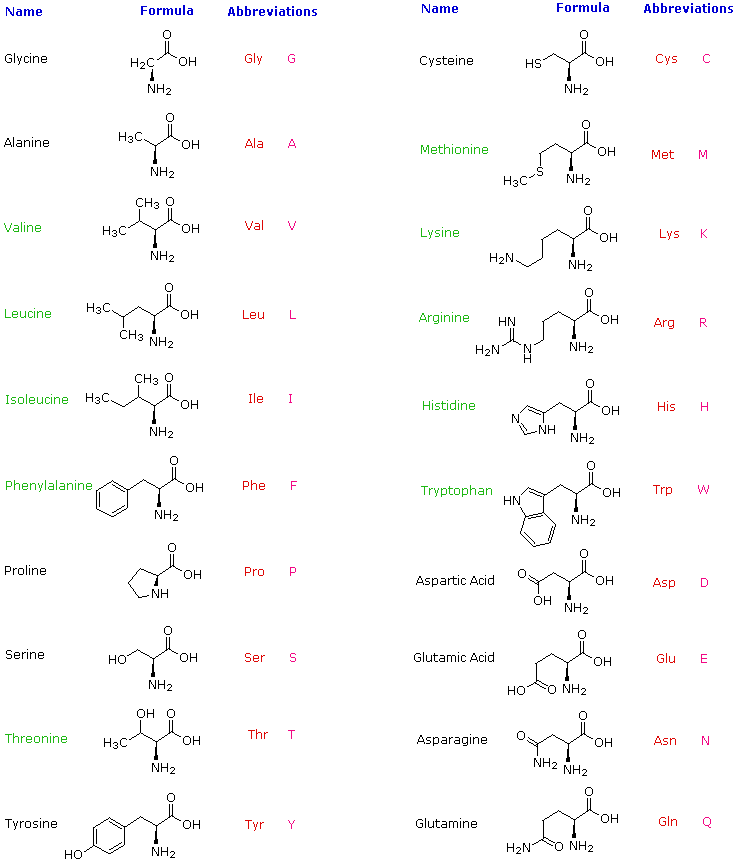
Amino Acids Peptides Pdf Docsity

Biochemistry Charge Of An Amino Acid Sequence Chemistry Stack
Another amino acid has a positively charged n terminus and a neutral c terminus at a low ph.

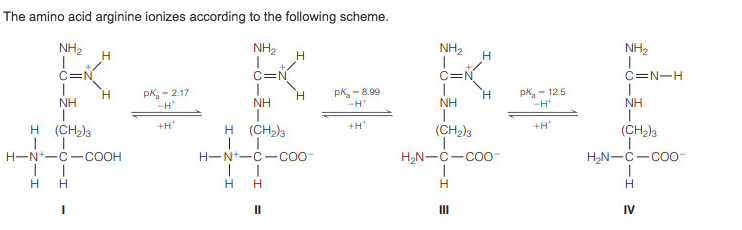
Arginine net charge. There will be no charge at the carboxy and a positive charge at the nitrogen for a net charge of 1. When we raise the ph a few units above the first pka and still well below the second pka value the carboxyl group will lose its proton. However i am not getting the right answer.
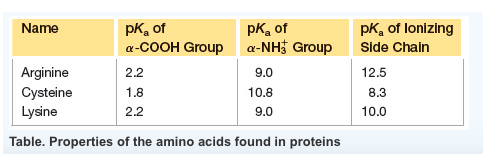
Lysines side chain amino group at the end of its side chain has a pka of about 9 10 so at ph 14 it will be deprotonated 0 charge therefore the net charge on lysine at ph14 is 1 0 0 1. Determining net charge of amino acid at given ph. Arginine has a net charge of 083 at ph 132 and am supposed to support this using the h h equation.
Doing the same for the side chains. As the ph rises to between 18 to 6 the amino acid has a net positive charge as carboxylic acid group becomes deprotonated and the terminal amino group and amino side chain remain protonated. Among the 20 common amino acids five have a side chain which can be charged.
1 so this protein should have a net charge of zero as all the positive and negative charges cancel out. Lysine lys k arginine arg r and histidine his h basic side chains. At physiological ph the carboxylic acid is deprotonated coo the amino group is protonated nh 3 and the guanidino group is also protonated to give the guanidinium form c nh 2 2 making arginine a charged aliphatic amino acid.
As ph increases the positively charged n terminus becomes neutral while the neutral c terminus becomes negatively charged. Aspartic acid asp d and glutamic acid glu e acidic side chains and three are positive charged. So the amino group will be protonated with a 1 charge and the carboxyl group will be deprotonated with a 1 charge.
Pka1 cooh 228 pka2 nh3 921 pi 574 from what i understand if ph pi which it is here the amino acid should have a negative charge because even though the ph is high enough to deprotonate all the carboxyl ends and not enough to deprotonate all the amino ends. However the amino group is still protonated. It is the precursor for the biosynthesis of nitric oxide.
When an amino acid aa is incorporated into a polypeptide the charges on the amino and carboxyl groups disappear.
19 13 Amino Acids Chemistry Libretexts

Agmatine An Overview Sciencedirect Topics
Solved The Optimum Ph To Separate A Mixture Of Lysine Ar

EmoticonEmoticon