This type of titration majorly depends on the track change in ph or a ph meter. The resultant difference curve is a convolution of three single site binding isotherms.

Solved 14 0 12 0 10 0 8 0 6 0 4 0 2 0 14 0 12 0 3 1 5pts

10 Glutamic Acid 325 Serine 57 Histidine 758 Lysine 975 Tyrosine 565
Announcements
Consider the ionization of a weak organic acid such as acetic acid by naoh.
Arginine titration curve. A titration curve of an amino acid is a plot of the ph of a weak acid against the degree of neutralization of the acid by standard strong base. 3 will be deprotonated. Titration of alanine with hydroxide on the left in both the chemical reaction and the titration curve you should imagine that alanine is in a very acidic solution at a ph of about 0.
Titration curve of lysine. 5 as the ph increases the second group nh. You have to use each amino acid in this experiment and must have a different side chain chemistry.
Titration curve of amino acid glycine 4 further increase in ph the solution will predominantly contains zwitter ion and the ph at this point is equal to pi. As you move from left to right accross the page you are adding hydroxide to the solution. Consider the ionization of a weak organic acid such as acetic acid by naoh.
In this type a reagent is mixed with the sample solution until it reached the required ph level. This result is from multiple experiments performed over the course of five months with sam ples containing 500 mm arginine. One for the guanidinium group of the arginine one for the lysine side chain z aminium and one for the lysine a aminium group.
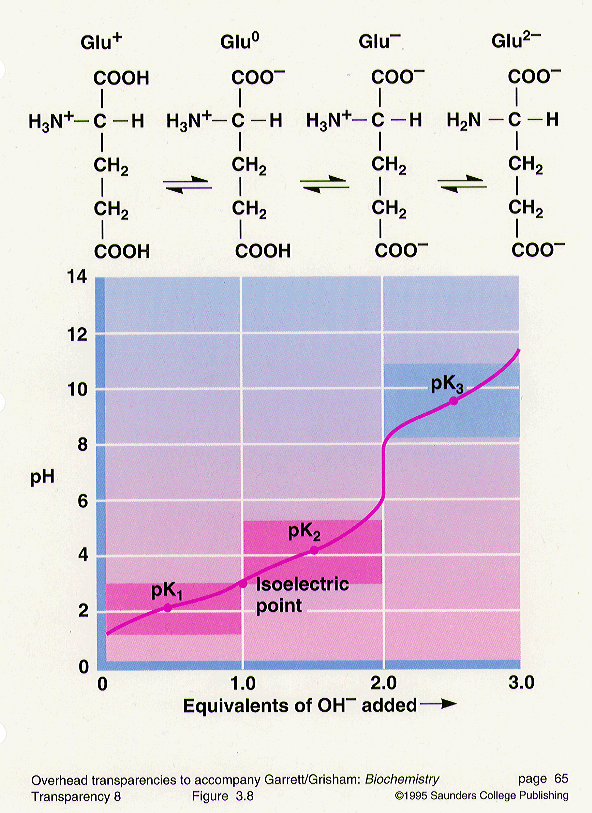
A titration curve of an amino acid is a plot of the ph of a weak acid against the degree of neutralization of the acid by standard strong base. Glutamine does not compose a titratable r group and therefore result in a production of distinct titration curve signature from all of the other amino acids who have constituted three titratable groups. A value of the arginine side chain.
The acid base titration is based on the reaction that neutralization between a base or an acidic and analyte. The difference curves were calculated by first interpolating the titration curves of both the arginine solution and the lysine solution followed by their subtraction at equivalent ph values. A value of the arginine guanidinium group obtained by analysis of the difference poten tiometric titration curves is on average 138601 table i.
Arginine Titration Curve Image Information
Arginine Its Pka Value Revisited
Arginine Titration Curve Traffic Club

EmoticonEmoticon